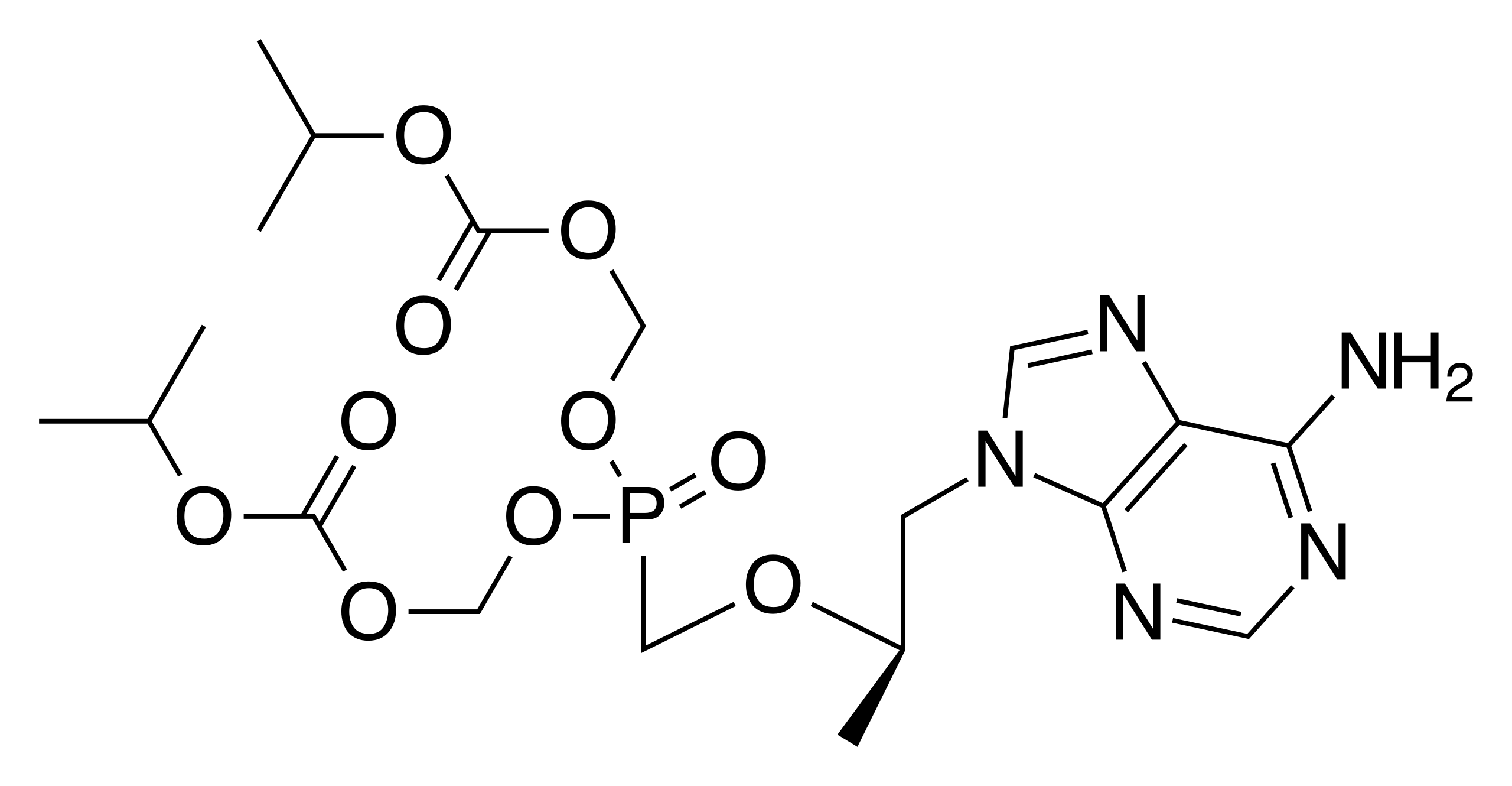
Tenofovir disoproxil fumarate (10g) (TDF-10g)
- Description
Description
Tenofovir disoproxil fumarate (tenofovir DF) is a type of anti-HIV medicine called a nucleoside reverse transcriptase inhibitor (NRTI).
MDL number MFCD00952920
Cas Number : 201341-05-1
Molecular Weight : 519.4400
Tenofovir disoproxil is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together as emtricitabine/tenofovir and efavirenz/emtricitabine/tenofovir.
[1] R. Salimi, I. Begum, D.M. Varma, B. Nandakrishna, R. Rajesh, S. Vidyasagar, Tenofovir disoproxil fumarate-induced distal renal tubular acidosis: A case report, Int J STD AIDS 31(3) (2020) 276-279.
[2] W.R. Zhang, R. Scherzer, M.M. Estrella, S.B. Ascher, A. Muiru, V. Jotwani, C. Grunfeld, C.R. Parikh, D. Gustafson, S. Kassaye, A. Sharma, M. Cohen, P.C. Tien, D.K. Ng, F.J. Palella, Jr., M.D. Witt, K. Ho, M.G. Shlipak, Tenofovir disoproxil fumarate initiation and changes in urinary biomarker concentrations among HIV-infected men and women, AIDS 33(4) (2019) 723-733.
[3] S. Noe, S. Heldwein, H. Jaeger, M. Page, E. Wolf, Tenofovir disoproxil fumarate/emtricitabine is associated with a higher risk of hypocalcemia compared to abacavir/lamivudine – results from a German cohort study, Int J STD AIDS 30(5) (2019) 447-452.
[4] M. Lu, H. Dong, D. Bao, B. Liu, H. Liu, Tenofovir disoproxil fumarate induces pheochromocytoma cells apoptosis, Eur J Pharmacol 844 (2019) 139-144.
[5] K. Liu, J. Choi, A. Le, T.C. Yip, V.W. Wong, S.L. Chan, H.L. Chan, M.H. Nguyen, Y.S. Lim, G.L. Wong, Tenofovir disoproxil fumarate reduces hepatocellular carcinoma, decompensation and death in chronic hepatitis B patients with cirrhosis, Aliment Pharmacol Ther 50(9) (2019) 1037-1048.
[6] M.J. Keller, L. Wood, J.M. Billingsley, L.L. Ray, J. Goymer, S. Sinclair, A.P. McGinn, M.A. Marzinke, B. Frank, S. Srinivasan, C. Liu, J.M. Atrio, L. Espinoza, N. Mugo, H.M.L. Spiegel, P.L. Anderson, D.N. Fredricks, C.W. Hendrix, J. Marrazzo, S.E. Bosinger, B.C. Herold, Tenofovir disoproxil fumarate intravaginal ring for HIV pre-exposure prophylaxis in sexually active women: a phase 1, single-blind, randomised, controlled trial, Lancet HIV 6(8) (2019) e498-e508.
[7] S. Kaneko, M. Kurosaki, N. Tamaki, J. Itakura, T. Hayashi, S. Kirino, L. Osawa, K. Watakabe, M. Okada, W. Wang, T. Shimizu, M. Higuchi, K. Takaura, Y. Yasui, K. Tsuchiya, H. Nakanishi, Y. Takahashi, M. Watanabe, N. Izumi, Tenofovir alafenamide for hepatitis B virus infection including switching therapy from tenofovir disoproxil fumarate, J Gastroenterol Hepatol 34(11) (2019) 2004-2010.
[8] R. Guner, Z. Kocak Tufan, G.R. Yilmaz, A.T. Mehmet, Tenofovir disoproxil fumarate may not cause renal and bone toxicity in chronic hepatitis B patients: a retrospective cross-sectional study, Turk J Med Sci 49(1) (2019) 451-452.
[9] J.A. Fields, M.K. Swinton, A. Carson, B. Soontornniyomkij, C. Lindsay, M.M. Han, K. Frizzi, S. Sambhwani, A. Murphy, C.L. Achim, R.J. Ellis, N.A. Calcutt, Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice, Sci Rep 9(1) (2019) 17158.
[10] J. Cusato, A. Calcagno, A. De Nicolo, K. Mogyorosi, A. D’Avolio, G. Di Perri, S. Bonora, Tenofovir Alafenamide and Tenofovir Disoproxil Fumarate are not transported by Concentrative Nucleoside Transporter 2, Diagn Microbiol Infect Dis 94(2) (2019) 202-204.
[11] A. Calcagno, M. Fiumano, D. Zugna, J. Cusato, C. Montrucchio, L. Marinaro, L. Trentini, M. Ferrara, A. D’Avolio, C. Pizzi, G. Di Perri, S. Bonora, Tenofovir disoproxil fumarate discontinuation for renal outcomes: any room for treatment personalization?, Pharmacogenomics J 19(1) (2019) 65-71.
[12] P. Zhang, Q. Liu, M. Yuan, L. Wang, Tenofovir disoproxil fumarate reduce incidence of HCC development in CHB-patients with compensated cirrhosis, Infect Agent Cancer 13 (2018) 30.
[13] R.R. Patel, R. Presti, L.C. Harrison, W.G. Powderly, P.A. Chan, Tenofovir disoproxil fumarate as pre-exposure prophylaxis for HIV prevention in women with osteoporosis: a case report and review of the literature, Antivir Ther 23(4) (2018) 379-382.
[14] S. Noe, S. Heldwein, C. Wiese, R. Pascucci, A. von Krosigk, F. Schabaz, C. Jonsson-Oldenbuettel, H. Jaeger, E. Wolf, Tenofovir Disoproxil Fumarate Is Associated with a Set-Point Variation in the Calcium-Parathyroid Hormone-Vitamin D Axis: Results from a German Cohort, Adv Pharmacol Sci 2018 (2018) 6069131.
[15] D. Mizushima, D.T.H. Nguyen, D.T. Nguyen, S. Matsumoto, J. Tanuma, H. Gatanaga, N.V. Trung, N. van Kinh, S. Oka, Tenofovir disoproxil fumarate co-administered with lopinavir/ritonavir is strongly associated with tubular damage and chronic kidney disease, J Infect Chemother 24(7) (2018) 549-554.
[16] S.H. Lee, G.J. Cheon, H.S. Kim, S.G. Kim, Y.S. Kim, S.W. Jeong, J.Y. Jang, B.S. Kim, B.G. Jun, Y. Don Kim, D.W. Jun, J.H. Sohn, T.Y. Kim, B.S. Lee, Tenofovir disoproxil fumarate monotherapy is superior to entecavir-adefovir combination therapy in patients with suboptimal response to lamivudine-adefovir therapy for nucleoside-resistant HBV: a 96-week prospective multicentre trial, Antivir Ther 23(3) (2018) 219-227.
[17] A.P. Kourtis, J. Wiener, L. Wang, B. Fan, J.A. Shepherd, L. Chen, W. Liu, C. Shepard, L. Wang, A. Wang, M. Bulterys, Tenofovir Disoproxil Fumarate Use during Pregnancy and Infant Bone Health: the Tenofovir in Pregnancy Pilot Study, Pediatr Infect Dis J 37(11) (2018) e264-e268.
[18] M.K. Kang, J.G. Park, Tenofovir disoproxil fumarate-induced severe liver injury in a patient with chronic hepatitis B virus infection, Dig Liver Dis 50(6) (2018) 628-630.
[19] A. Hill, S.L. Hughes, D. Gotham, A.L. Pozniak, Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?, J Virus Erad 4(2) (2018) 72-79.
[20] P.L. Havens, D. Long, G.U. Schuster, C.M. Gordon, G. Price, C.M. Wilson, B.G. Kapogiannis, K. Mulligan, C.B. Stephensen, H.I.V.A.I. Adolescent Medicine Trials Network for, t. study, Tenofovir disoproxil fumarate appears to disrupt the relationship of vitamin D and parathyroid hormone, Antivir Ther 23(7) (2018) 623-628.
[21] T.R. Cressey, L. Harrison, J. Achalapong, P. Kanjanavikai, O. Patamasingh Na Ayudhaya, P. Liampongsabuddhi, T. Siriwachirachai, C. Putiyanun, P. Suriyachai, C. Tierney, N. Salvadori, D. Chinwong, L. Decker, Y. Tawon, T.V. Murphy, N. Ngo-Giang-Huong, G.K. Siberry, G. Jourdain, T.A.P.S.T. i, Tenofovir Exposure during Pregnancy and Postpartum in Women Receiving Tenofovir Disoproxil Fumarate for the Prevention of Mother-to-Child Transmission of Hepatitis B Virus, Antimicrob Agents Chemother 62(12) (2018).
[22] W. Tun-Yhong, C. Chinpaisal, P. Pamonsinlapatham, S. Kaewkitichai, Tenofovir Disoproxil Fumarate Is a New Substrate of ATP-Binding Cassette Subfamily C Member 11, Antimicrob Agents Chemother 61(4) (2017).
[23] H. Shen, W. Li, W.G. Humphreys, Y. Lai, Tenofovir Disoproxil Fumarate Is Not an Inhibitor of Human Organic Cation Transporter 1, J Pharmacol Exp Ther 360(2) (2017) 341-342.
[24] J. Shailender, P.R. Ravi, P. Saha, A. Dalvi, S. Myneni, Tenofovir disoproxil fumarate loaded PLGA nanoparticles for enhanced oral absorption: Effect of experimental variables and in vitro, ex vivo and in vivo evaluation, Colloids Surf B Biointerfaces 158 (2017) 610-619.
[25] A.A. Shaheen, M. AlMattooq, S. Yazdanfar, K.W. Burak, M.G. Swain, S.E. Congly, M.A. Borman, S.S. Lee, R.P. Myers, C.S. Coffin, Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers, Aliment Pharmacol Ther 46(6) (2017) 599-604.
[26] R. Samuels, C.R. Bayerri, J.A. Sayer, D.A. Price, B.A.I. Payne, Tenofovir disoproxil fumarate-associated renal tubular dysfunction: noninvasive assessment of mitochondrial injury, AIDS 31(9) (2017) 1297-1301.
[27] F.A. Post, L. Hamzah, J. Fox, Tenofovir disoproxil fumarate-associated bone loss: does vitamin D-binding protein play a role?, AIDS 31(1) (2017) 178-179.
[28] L.M. Mofenson, R.C. Baggaley, I. Mameletzis, Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding, AIDS 31(2) (2017) 213-232.
[29] S. Khan, C.A. Funk, K. Corado, S. Morris, M.P. Dube, Tenofovir Disoproxil Fumarate-Associated Fanconi Syndrome in an Human Immunodeficiency Virus (HIV)-Uninfected Man Receiving HIV Pre-Exposure Prophylaxis, Open Forum Infect Dis 4(3) (2017) ofx149.
[30] A. Inciarte, L. Leal, E. Gonzalez, A. Leon, C. Lucero, J. Mallolas, B. Torres, M. Laguno, J. Rojas, M. Martinez-Rebollar, A. Gonzalez-Cordon, A. Cruceta, J.A. Arnaiz, J.M. Gatell, F. Garcia, S.S. Group, Tenofovir disoproxil fumarate/emtricitabine plus ritonavir-boosted lopinavir or cobicistat-boosted elvitegravir as a single-tablet regimen for HIV post-exposure prophylaxis, J Antimicrob Chemother 72(10) (2017) 2857-2861.
[31] Y.C. Hsu, M.T. Wei, M.H. Nguyen, Tenofovir alafenamide as compared to tenofovir disoproxil fumarate in the management of chronic hepatitis B with recent trends in patient demographics, Expert Rev Gastroenterol Hepatol 11(11) (2017) 999-1008.
[32] S. Fung, P. Kwan, M. Fabri, A. Horban, M. Pelemis, H.W. Hann, S. Gurel, F.A. Caruntu, J.F. Flaherty, B. Massetto, K. Kim, K.M. Kitrinos, G.M. Subramanian, J.G. McHutchison, L.J. Yee, M. Elkhashab, T. Berg, I. Sporea, C. Yurdaydin, P. Husa, M.S. Jablkowski, E. Gane, Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study, J Hepatol 66(1) (2017) 11-18.
[33] G. Ruggiero, A. Marrone, I. Rainone, A. Boemio, L.E. Adinolfi, G. Pasquale, L. Rinaldi, B. Guerrera, L. Andreana, R. Zampino, G. Thecla Study, Tenofovir disoproxil fumarate monotherapy maintains HBV suppression achieved by a “de novo” combination of lamivudine-adefovir: a pilot study, Infez Med 24(4) (2016) 278-286.
[34] P. Penot, C. Gosset, J. Verine, J.M. Molina, Tenofovir disoproxil fumarate-induced Fanconi’s syndrome during HIV postexposure prophylaxis, AIDS 30(8) (2016) 1311-3.
[35] J. Fox, M. Brady, H. Alexander, O. Davies, N. Robinson, M. Pace, L. Else, J. Cason, S. Khoo, D. Back, S. Fidler, J. Frater, Tenofovir Disoproxil Fumarate Fails to Prevent HIV Acquisition or the Establishment of a Viral Reservoir: Two Case Reports, Infect Dis Ther 5(1) (2016) 65-71.
[36] E. De Clercq, Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF), Biochem Pharmacol 119 (2016) 1-7.
[37] C.Y. Cheng, S.Y. Chang, M.H. Lin, S.Y. Ku, N.L. Sun, S.H. Cheng, Tenofovir disoproxil fumarate-associated hypophosphatemia as determined by fractional excretion of filtered phosphate in HIV-infected patients, J Infect Chemother 22(11) (2016) 744-747.
[38] H.L. Chan, S. Fung, W.K. Seto, W.L. Chuang, C.Y. Chen, H.J. Kim, A.J. Hui, H.L. Janssen, A. Chowdhury, T.Y. Tsang, R. Mehta, E. Gane, J.F. Flaherty, B. Massetto, A. Gaggar, K.M. Kitrinos, L. Lin, G.M. Subramanian, J.G. McHutchison, Y.S. Lim, S.K. Acharya, K. Agarwal, G.-U.-. Investigators, Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial, Lancet Gastroenterol Hepatol 1(3) (2016) 185-195.
[39] M. Buti, E. Gane, W.K. Seto, H.L. Chan, W.L. Chuang, T. Stepanova, A.J. Hui, Y.S. Lim, R. Mehta, H.L. Janssen, S.K. Acharya, J.F. Flaherty, B. Massetto, A.L. Cathcart, K. Kim, A. Gaggar, G.M. Subramanian, J.G. McHutchison, C.Q. Pan, M. Brunetto, N. Izumi, P. Marcellin, G.-U.-. Investigators, Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial, Lancet Gastroenterol Hepatol 1(3) (2016) 196-206.
[40] D.H. Yang, Y.J. Xie, N.F. Zhao, H.Y. Pan, M.W. Li, H.J. Huang, Tenofovir disoproxil fumarate is superior to lamivudine plus adefovir in lamivudine-resistant chronic hepatitis B patients, World J Gastroenterol 21(9) (2015) 2746-53.
[41] J.M. Smith, P. Srinivasan, R.S. Teller, Y. Lo, C.T. Dinh, P.F. Kiser, B.C. Herold, Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures, J Acquir Immune Defic Syndr 68(1) (2015) 1-5.
[42] P.E. Sax, D. Wohl, M.T. Yin, F. Post, E. DeJesus, M. Saag, A. Pozniak, M. Thompson, D. Podzamczer, J.M. Molina, S. Oka, E. Koenig, B. Trottier, J. Andrade-Villanueva, G. Crofoot, J.M. Custodio, A. Plummer, L. Zhong, H. Cao, H. Martin, C. Callebaut, A.K. Cheng, M.W. Fordyce, S. McCallister, G.-U.-S. Team, Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials, Lancet 385(9987) (2015) 2606-15.
[43] H.H. Ng, H. Stock, L. Rausch, D. Bunin, A. Wang, S. Brill, J. Gow, J.C. Mirsalis, Tenofovir disoproxil fumarate: toxicity, toxicokinetics, and toxicogenomics analysis after 13 weeks of oral administration in mice, Int J Toxicol 34(1) (2015) 4-10.
[44] A. Mills, G. Crofoot, Jr., C. McDonald, P. Shalit, J.A. Flamm, J. Gathe, Jr., A. Scribner, D. Shamblaw, M. Saag, H. Cao, H. Martin, M. Das, A. Thomas, H.C. Liu, M. Yan, C. Callebaut, J. Custodio, A. Cheng, S. McCallister, Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate in the First Protease Inhibitor-Based Single-Tablet Regimen for Initial HIV-1 Therapy: A Randomized Phase 2 Study, J Acquir Immune Defic Syndr 69(4) (2015) 439-45.
[45] S.K. Jung, K.A. Kim, S.Y. Ha, H.K. Lee, Y.D. Kim, B.H. Lee, W.H. Paik, J.W. Kim, W.K. Bae, N.H. Kim, J.S. Lee, Y.J. Jwa, Tenofovir disoproxil fumarate monotherapy for nucleos(t)ide analogue-naive and nucleos(t)ide analogue-experienced chronic hepatitis B patients, Clin Mol Hepatol 21(1) (2015) 41-8.
[46] J.L. Hou, Z.L. Gao, Q. Xie, J.M. Zhang, J.F. Sheng, J. Cheng, C.W. Chen, Q. Mao, W. Zhao, H. Ren, D.M. Tan, J.Q. Niu, S.J. Chen, C. Pan, H. Tang, H. Wang, Y.M. Mao, J.D. Jia, Q. Ning, M. Xu, S.M. Wu, J. Li, X.X. Zhang, Y. Ji, J. Dong, J. Li, Tenofovir disoproxil fumarate vs adefovir dipivoxil in Chinese patients with chronic hepatitis B after 48 weeks: a randomized controlled trial, J Viral Hepat 22(2) (2015) 85-93.
[47] S. Fung, S.C. Gordon, Z. Krastev, A. Horban, J. Petersen, J. Sperl, E. Gane, I.M. Jacobson, L.J. Yee, P. Dinh, E.B. Martins, J.F. Flaherty, K.M. Kitrinos, G. Dusheiko, H. Trinh, R. Flisiak, V.K. Rustgi, M. Buti, P. Marcellin, Tenofovir disoproxil fumarate in Asian or Pacific Islander chronic hepatitis B patients with high viral load (>/= 9 log10 copies/ml), Liver Int 35(2) (2015) 422-8.
[48] B. Baran, O.M. Soyer, A.C. Ormeci, S. Gokturk, S. Evirgen, F. Akyuz, C. Karaca, K. Demir, F. Besisik, D. Onel, M. Gulluoglu, S. Badur, S. Kaymakoglu, Tenofovir disoproxil fumarate has a substantial efficacy against multidrug-resistant strains of hepatitis B virus, Liver Int 35(10) (2015) 2265-74.
[49] C. Ayaz, M.K. Celen, T. Dal, O. Deveci, K. Bayan, D. Mert, E. Oruc, N. Ozcan, I. Kandemir, M.S. Dal, Tenofovir disoproxil fumarate treatment in HbeAg-positive patients, Infez Med 23(1) (2015) 31-5.
[50] P.E. Sax, A. Zolopa, I. Brar, R. Elion, R. Ortiz, F. Post, H. Wang, C. Callebaut, H. Martin, M.W. Fordyce, S. McCallister, Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study, J Acquir Immune Defic Syndr 67(1) (2014) 52-8.
[51] W. Rao, M. Xie, Z. Shen, Tenofovir disoproxil fumarate rescue therapy for HBV recurrence in two liver transplant recipients with previous multiple nucleo(s/t)ide treatment failures, Transpl Int 27(10) (2014) e102-4.
[52] S.S. Ahn, Y.E. Chon, B.K. Kim, S.U. Kim, D.Y. Kim, S.H. Ahn, K.H. Han, J.Y. Park, Tenofovir disoproxil fumarate monotherapy for nucleos(t)ide-naive chronic hepatitis B patients in Korea: data from the clinical practice setting in a single-center cohort, Clin Mol Hepatol 20(3) (2014) 261-6.
[53] K. Woratanarat, T. Kanjanabuch, C. Suankratay, Tenofovir disoproxil fumarate-associated nephrotoxicity in HIV-infected patients: a prospective controlled study, J Med Assoc Thai 96(4) (2013) 432-9.
[54] W.K. Seto, M.F. Yuen, J. Fung, C.L. Lai, Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B monoinfection, Hepatol Int 7(2) (2013) 327-34.
[55] C.Q. Pan, L.J. Mi, C. Bunchorntavakul, J. Karsdon, W.M. Huang, G. Singhvi, M.G. Ghany, K.R. Reddy, Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series, Dig Dis Sci 57(9) (2012) 2423-9.
[56] J.A. Moss, M.M. Baum, A.M. Malone, S. Kennedy, E. Kopin, C. Nguyen, J. Gilman, I. Butkyavichene, R.A. Willis, K.L. Vincent, M. Motamedi, T.J. Smith, Tenofovir and tenofovir disoproxil fumarate pharmacokinetics from intravaginal rings, AIDS 26(6) (2012) 707-10.
[57] M. Buti, M. Homs, Tenofovir disoproxil fumarate in the treatment of chronic hepatitis B, Expert Rev Gastroenterol Hepatol 6(4) (2012) 413-21.
[58] S.J. Patterson, J. George, S.I. Strasser, A.U. Lee, W. Sievert, A.J. Nicoll, P.V. Desmond, S.K. Roberts, S. Locarnini, S. Bowden, P.W. Angus, Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B, Gut 60(2) (2011) 247-54.
[59] Y.F. Liaw, I.S. Sheen, C.M. Lee, U.S. Akarca, G.V. Papatheodoridis, F. Suet-Hing Wong, T.T. Chang, A. Horban, C. Wang, P. Kwan, M. Buti, M. Prieto, T. Berg, K. Kitrinos, K. Peschell, E. Mondou, D. Frederick, F. Rousseau, E.R. Schiff, Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease, Hepatology 53(1) (2011) 62-72.
[60] A. Vigano, G.V. Zuccotti, M. Puzzovio, V. Pivetti, I. Zamproni, C. Cerini, V. Fabiano, V. Giacomet, S. Mora, Tenofovir disoproxil fumarate and bone mineral density: a 60-month longitudinal study in a cohort of HIV-infected youths, Antivir Ther 15(7) (2010) 1053-8.
[61] J. Jones, J. Colquitt, J. Shepherd, P. Harris, K. Cooper, Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection, Health Technol Assess 14 Suppl 1 (2010) 23-9.
[62] A.M. Jenh, P.A. Pham, Tenofovir disoproxil fumarate in the treatment of chronic hepatitis B, Expert Rev Anti Infect Ther 8(10) (2010) 1079-92.
[63] C.M. Perry, D. Simpson, Tenofovir disoproxil fumarate: in chronic hepatitis B, Drugs 69(16) (2009) 2245-56.
[64] C. Foster, H. Lyall, B. Olmscheid, G. Pearce, S. Zhang, D.M. Gibb, Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine?, HIV Med 10(7) (2009) 397-406.
[65] S. Adusumilli, Tenofovir disoproxil fumarate for the treatment of hepatitis B infection, Drugs Today (Barc) 45(9) (2009) 679-85.
[66] P. Marcellin, E.J. Heathcote, M. Buti, E. Gane, R.A. de Man, Z. Krastev, G. Germanidis, S.S. Lee, R. Flisiak, K. Kaita, M. Manns, I. Kotzev, K. Tchernev, P. Buggisch, F. Weilert, O.O. Kurdas, M.L. Shiffman, H. Trinh, M.K. Washington, J. Sorbel, J. Anderson, A. Snow-Lampart, E. Mondou, J. Quinn, F. Rousseau, Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B, N Engl J Med 359(23) (2008) 2442-55.
[67] J.R. Arribas, A.L. Pozniak, J.E. Gallant, E. Dejesus, B. Gazzard, R.E. Campo, S.S. Chen, D. McColl, C.B. Holmes, J. Enejosa, J.J. Toole, A.K. Cheng, Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis, J Acquir Immune Defic Syndr 47(1) (2008) 74-8.
[68] L. Peterson, D. Taylor, R. Roddy, G. Belai, P. Phillips, K. Nanda, R. Grant, E.E. Clarke, A.S. Doh, R. Ridzon, H.S. Jaffe, W. Cates, Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial, PLoS Clin Trials 2(5) (2007) e27.